Abstract
Introduction: Acute myeloid leukaemia (AML) is a heterogeneous disorder that arises from clonal expansion of malignant hematopoietic precursor cells. Somatic mutations of the p53 gene have been reported in 5-10% of AML, with a higher incidence therapy-related disease and elderly patients. Alteration or loss of p53 is one of the most powerful independent indicators of poor outcome.
Methods: This is a retrospective analysis of AML & high risk-Myelodysplastic syndrome (HR-MDS) patients treated over the study period (2006-2020). Informed consent was obtained. Initial presentation, treatment response, and survival were analysed. Percentage of p53 expression (a surrogate marker for TP53 mutations) by immunohistochemistry (IHC) (>30% cut-off) on BM trephines was analysed and its impact on treatment outcome was evaluated.
Results: We identified 114 patients (AML=104 & HR-MDS=10), the median age was 70 years (Range 36-85) with male predominance (M=73 vs. F=41). The AML group included, 57 patients (50%) with Denovo AML and 47 patients (41%) with Secondary AML (MDS=27, MPN=12 / JAKII mutation =7, t-AML=8). The median age was 70 years (Range 36-85) and the median blast count was 70% (Range 25-95%). Cytogenetic analysis reports were available on 78 patients (75%)(Poor risk=40 vs. Intermediate risk=38). NPM1 (22 patients) and FLT3 mutations analysis (33 patients )were available and reported as positive in 4(18%) and 5(15%) patients respectively.
The HR-MDS is defined by patients who fall into higher-risk group categories in the original or revised IPSS (N=10), the median age was 74 years (Range 63-83) with male predominance (M=7 vs. F=3). The median blast count was 15% (Range 11-18%). Cytogenetic risk prognostic subgroup results were available on 6 patients (60%), 2 patients (2%) had intermediate-risk and 4 patients (4%) had poor-risk.
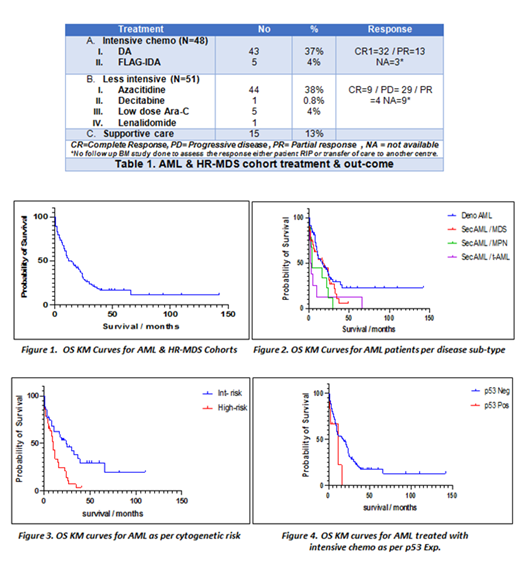
Ninety-nine patients (87%) were eligible for treatment, 48 patients (42%) were treated with intensive induction chemotherapy, and 51 patients (45%) received less intensive therapy (HMA / LD-Ara C). Five patients (4%) consolidated with allogeneic stem cell transplant (Allo-SCT) post-induction therapy. The remaining (15 patients) were on supportive care. Forty-one AML patients (36%) relapsed post CR1, 3 patients had Allo-SCT in CR2. Table 1
At the time of study analysis (31/01/2021), 25 patients (22%) are still alive including 23 patients (20%) with AML (Denovo AML=18 & Sec AML=5) and 2 patients (4%) with HR-MDS. The median age of the survival group was 69 years (Range 51-83). The whole study cohort median OS was 12 months. (Figure. 1) The median OS & PFS for AML patients were 15 & 13 months respectively. According to AML sub-type, t-AML associated with shortest median OS of 2.5 months (P.value 0.0190). (Figure. 2) The median OS was more favourable for intermediate-risk than the high-risk cytogenetic AML, 24 and 10 months respectively (P<00016).(Figure. 3) The HR-MDS group median OS was 22 months.
One hundred and four patient's BM trephines (91%) were available and screened for p53 IHC expression (AML= 96 & HR-MDS =8). Eight samples (8%) showed p53 over-expression, all these samples with underlying AML diagnosis. The remaining 96 samples (92%) were negative for high p53 expression. The majority of p53 over-expressed AML harboured complex cytogenetics, 4 patients with Sec AML, 3 patients with Denovo AML, and 1 patient with t-AML. Most of the patients (5 patients) in this sub-set had a refractory disease to induction therapy, of whom 2 patients (25%) had Allo-SCT consolidation in CR2. Only one patient (12%) is still alive in this sub-group.
The median OS & PFS in p53 wild-type AML cohort was 16 & 13 months respectively, which compared favourably to 12 & 8 months in those with p53 disruptions. The P. values were 0.134 & 0.193, respectively. The impact of the p53 alterations was more pronounced on the AML sub-group treated with intensive chemotherapy (42%), the median OS & PFS in wild-type p53 AML were 24 & 20 months, while in p53 disrupted cohort were 12 & 10 months respectively (P= 0.030)(95%CI of ratio 0.1755 to 1.425) & (P=0.049)( 95%CI of ratio 0.7015 to 5.702).(Figure.4)
Conclusion: In this study we demonstrated the potential role for p53 expression by IHC, which is readily available in routine practice, in assessing AML prognosis. Our real-life data confirms the dismal prognosis of p53 alterations in this disease. Participation in clinical trials based on genetic risk stratification is warranted.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal